FDA and CDC Recommend Pausing J&J Vaccine Amid Reports of Rare Blood Clots
The agencies say they’re making the move out of an abundance of caution
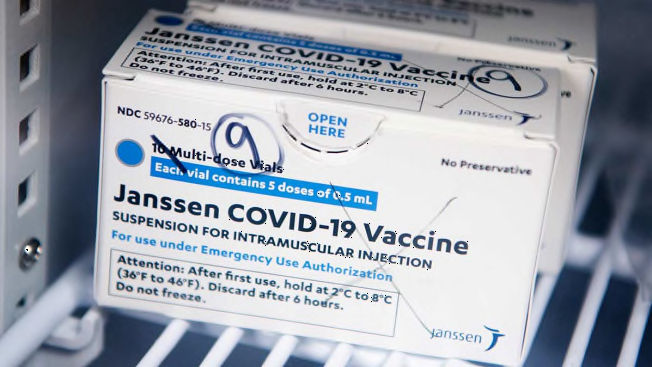
The Food and Drug Administration and the Centers for Disease Control and Prevention on Tuesday recommended pausing the use of the COVID-19 vaccine made by Johnson & Johnson while both agencies review reports of six cases of rare and severe blood clots that have occurred in women who received that vaccine.
So far, more than 6.8 million doses of that vaccine have been given in the U.S, making these events extremely rare, according to Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, who spoke at a joint FDA and CDC press conference this morning. But the agencies recommended pausing as they educate healthcare providers about how to treat these clots. Notably, while some other blood clots are commonly treated with heparin, a blood thinner, that drug should not be used with these blood clots, Marks said.
“We’re recommending this pause while we work together to fully understand these events, and also so we can get information out to healthcare providers and vaccine recipients,” Janet Woodcock, MD, acting FDA commissioner, said at the news conference.
What Should You Do If You’ve Received or Are Scheduled to Get the J&J Vaccine?
If you received the J&J vaccine a month or longer ago, there’s very little risk, Anne Schuchat, MD, principal deputy director for the CDC, said at this morning’s news conference. People who received that vaccine within the past couple of weeks should be aware of symptoms of these clots, she said. People who experience severe headaches, leg pain, abdominal pain, or shortness of breath should contact their healthcare provider and seek treatment.
If you are scheduled to get the J&J vaccine in the coming days, your next steps may depend on where you are and where you are scheduled to get vaccinated.
The advice from the CDC and the FDA is not an absolute order; individual doctors and patients can still opt to use the J&J vaccine if it’s available and if they determine that the benefit outweighs the risk. But many states have already said that for now they will stop using the vaccine in state-run vaccination programs, and several large pharmacy chains, including CVS and Walgreens, have also said they’ll stop using it.
Vaccine distributors that are working with the federal government are taking steps to reschedule people who have J&J vaccine appointments with appointments to get one of the other available vaccines, Schuchat said.
In some places, that may result in no change of plans for people heading out to be vaccinated—in New York, for example, state health commissioner Howard Zucker announced that all people scheduled to get the J&J vaccine today would receive the Pfizer vaccine. In other states, including Virginia, health officials have announced that people will be contacted to reschedule appointments as needed.
Next Steps
The FDA expects the pause to be in place for a matter of days, according to Woodcock. The CDC is convening a meeting of an independent advisory group on vaccines tomorrow to further discuss the next steps.
It’s likely that more of these cases could be discovered as officials investigate over the coming days, according to Marks.
As virologist Angela Rasmussen and many others have pointed out on Twitter, the risk of these clots appears to be extremely low, especially when compared with the much higher risk of clots with oral contraceptives, for example.
But if the FDA determines that the risk is higher for a certain group of people, that could change recommendations for vaccine usage going forward. In Canada, for example, the National Advisory Committee on Immunization has advised using the AstraZeneca vaccine only in adults 55 and older because the risk of clots appears to be higher in younger adults.