Sign In

Menu
Suggested Searches
Recent Searches
Suggested Searches
Product Ratings
Resources
Chat With AskCR
Resources
All Products A-ZThe payment for your account couldn't be processed or you've canceled your account with us.
Re-activateMy account
Sign In
My account
Sign In

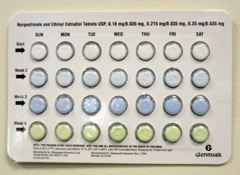
Glenmark Generics has recalled seven lots of its generic birth control pills Norgestimate and Ethinyl Estradiol, because in some blister packs the weekly tablet orientation is reversed, putting the pills in the wrong order.
The oral contraceptives were distributed nationwide between September 21 and December 30, 2011. The issue was discovered when a consumer told Glenmark that she got a blister pack in which the tablets were packaged in reverse order.
The pill order is reversed because the blister packaging is rotated 180 degrees within the card, which also makes the lot number and expiration date only visible on the outer pouch. Any blister pack in which the lot number and expiration date are not visible is subject to this recall.
Consumers exposed to affected packaging should begin using a non-hormonal form of contraception. Patients who have the affected blister packs should notify their physician and return the recalled pills to the pharmacy.
The lot numbers of recalled Norgestimate and Ethinyl Estradiol tablets (0.18 mg/0.035 mg, 0.215 mg/0.035 mg, 0.25 mg/0.035 mg) are as follows:
NDC: 68462-565-29
Adverse events related to this recall can be reported to Glenmark Generics at 888-721-7115 (8 a.m. to 5 p.m. Mon-Fri EST) or to the Food and Drug Administration's MedWatch Program.
—Maggie Shader
 Build & Buy Car Buying Service
Build & Buy Car Buying Service
Save thousands off MSRP with upfront dealer pricing information and a transparent car buying experience.
 Get Ratings on the go and compare
Get Ratings on the go and compare
while you shop