Sign In

The medications used to treat mental decline in people with Alzheimer's disease are not particularly effective. When compared to a placebo, most people who take one will not experience a meaningful benefit. And it is the rare person who has a significant delay in the worsening of their symptoms over time.
However, there is no way to predict who will get a benefit from one of the five drugs approved to treat Alzheimer's disease and who will not. So the decision to try one of these drugs should be based on whether any potential benefit is worth the cost, and the risk of side effects.
Because most people who take an Alzheimer's medication will receive no meaningful benefit, together with the relatively high price tag and the risk of rare but important safety concerns, we are unable to choose any of these drugs as a Best Buy. However, we realize that many people will want to try one of these medications if somebody they care for is suffering from Alzheimer's disease.
In that case, it makes sense to try one that has the lowest rate of side effects and is the least expensive since none of the medications has been shown to be more effective than the others. Generic donepezil or generic galantamine meet both criteria. Both have a lower risk of adverse effects and higher tolerability than the other medications, and since they are generic, their price is significantly less. But if the person taking the drug does not show signs of improvement within three months, it is unlikely that they ever will, so the drug should then be stopped.

Alzheimer's disease is the most common cause of dementia, the medical term for a decline in memory, thinking, decision-making, and reasoning. As many as 5.1 million Americans have Alzheimer's disease, according to the National Institute on Aging. That number is projected to substantially increase in the coming years because the U.S. population is aging, which increases the risk of developing Alzheimer's.
Despite decades of research, it remains unclear what causes Alzheimer's. What is known is that once the disease begins, it causes progressive destruction of brain cells, which leads to a decline in memory and cognitive functioning, as well as changes in personality and behavior. Research indicates that Alzheimer's can run in families, and the tendency to get it is inherited. However, even if you have a family history of Alzheimer's, that does not mean you will get it. Studies suggest, for example, that regular physical and mental activity that keeps your mind engaged — such as doing crossword puzzles or playing bridge — as well as strong social ties and personal relationships, may help to prevent its onset.
Smoking, high cholesterol, and diabetes increase the risk of developing dementia. Quitting smoking, and controlling high cholesterol and diabetes with a healthy lifestyle and proper treatment, which might include medication, can help reduce the risk of developing dementia.
Many people, as they age, worry they might be developing Alzheimer's disease when they start forgetting simple things, such as phone numbers, or when they start misplacing things, such as keys and eye glasses. Those problems are common and usually do not indicate any memory or brain disorder, even if they worsen as you age. Actually, the symptoms of Alzheimer's are quite distinct. Table 1 below gives you a quick assessment of the differences and the various stages of Alzheimer's disease. It typically progresses from early-stage disease characteristics, to middle-stage, to, finally, late-stage.
Table 1. The Stages of Alzheimer's Disease
| Not Alzheimer's | Early-stage |
|
|
| Middle-stage | Late-stage |
|
|
Sources: Multiple, including the Alzheimer's Association
In the early stages, symptoms and behavioral changes may be quite mild and minimally disruptive. Or only a few symptoms may be present, such as short-term memory loss. People with Alzheimer's eventually become unable to make and store new memories. The part of the brain (called the hippocampus) responsible for learning new information is one of the first to be damaged by the disease. This leads to a rapid forgetting of information, such as not recalling a conversation that happened just a few hours or even minutes ago. But people with Alzheimer's retain memories from long ago, prior to the beginning of the disease, such as details of one's wedding day or childhood teachers.
This early stage may last for up to a couple of years. But as time goes by, the symptoms usually worsen. As the disease progresses to the middle stages, a person may wander away from home, be unable to do even easy math or writing, have trouble getting dressed, and become delusional.
In the late stages of the disease, Alzheimer's patients usually can't communicate with words, are unable to bathe, eat, dress, or use the toilet, and may even lose the ability to swallow or chew. The personality changes also deepen, and some people with Alzheimer's become abusive, highly anxious, agitated, delusional, or paranoid. Alzheimer's is eventually a fatal disease, although many with the condition die of something else, such as pneumonia, and not the disease itself.
How it is diagnosed?
Especially in its early stages, Alzheimer's is not easy to diagnose. A diagnosis usually involves a series of oral and written tests that evaluate memory and thinking ability. You may also be asked to take blood or other tests to rule out other potential causes of dementia, such as Parkinson's disease or stroke, hypothyroidism, and vitamin B-12 deficiency (also called pernicious anemia). In addition, many medicines can produce mental and behavioral changes that mimic the symptoms of Alzheimer's disease.
It is also common for a doctor to order brain scans, such as a CT or MRI, to rule out other potential causes of dementia, including strokes and tumors. But those tests, at present, are unable to determine conclusively if a person with memory loss has Alzheimer's disease or another cause of dementia.
Differentiating older people who have depression versus early Alzheimer's can be a challenge, too. Depression occurs in up to half of people with Alzheimer's, and may, in fact, be more pronounced in the early stages when people are grappling with the onset of the disease. But, of course, seniors without Alzheimer's can also become clinically depressed. Thus, it's important that an older person who is suspected of having Alzheimer's or depression receive thorough testing and a definitive diagnosis.
Five drugs are currently approved by the Food and Drug Administration (FDA) to specifically treat Alzheimer's disease. They are:
| Generic Name | Brand Name(s) | Available as a Generic Drug? |
| Donepezil | Aricept Aricept dissolvable tablet |
Yes |
| Galantamine | Razadyne Razadyne ER |
Yes |
| Memantine | Namenda Namenda XR |
Yes* |
| Rivastigmine | Exelon | Yes |
| Tacrine | Cognex | No |
* A generic formulation of Namenda was approved by the FDA in March 2012, but at the time this report was published, the drug had not yet become available.
These five medicines are sometimes used to treat other conditions that can lead to dementia, such as Parkinson's disease. In this report, however, we focus on their use to treat people with Alzheimer's disease.
Exelon is also available as a patch, but this was not included in our analysis, so we can't say how it compares to the other medications. But the labeling notes that the most common side effects associated with the patch include nausea, vomiting, and diarrhea. It can also cause gastrointestinal bleeding, loss of appetite, and weight loss, and mistakes in using the patch have resulted in the need for hospitalization and rare cases of death.
Although many have been tried, no other types of medicines have been shown to be effective in delaying the onset of, or reducing, Alzheimer's cognitive symptoms and disabilities. This includes the anti-inflammatory drugs, such as ibuprofen (Advil, Motrin) and celecoxib (Celebrex); the Parkinson's disease drug, selegiline (Eldepryl); the cholesterol-lowering drugs known as statins; or a drug called piracetam.
Likewise, no studies have shown that alternative treatments work to delay the onset of Alzheimer's or help reduce its symptoms. This includes fish oil supplements; folic acid supplements; the herbal supplement, ginkgo biloba; the epilepsy drug, carbamazepine (Carbetrol, Tegretol); and vitamin C.
Female hormone drugs (estrogen and progestin) were once thought to lower the chances of memory decline and getting Alzheimer's. But studies have found that hormone replacement therapy is actually associated with an increased risk of dementia (mostly stroke-related but possibly also Alzheimer's) in women 65 and older. The science is not settled, however, and researchers are now investigating whether estrogen started earlier at the time of menopause has a protective effect.
It's important for you to know that medicines are but one component in the care of people with Alzheimer's. Studies indicate that psychosocial support and nonmedical treatment are just as important.
Doctors sometimes prescribe other medicines than those we focus on in this report—such as antidepressants, antipsychotics, and anxiety medications—to help control some of the behavioral problems that can occur in people with Alzheimer's. Antidepressants and anxiety drugs—used appropriately and judiciously—might be helpful. Antipsychotic drugs—such as risperidone (Risperdal), quetiapine (Seroquel), and olanzapine (Zyprexa)—are sometimes prescribed to help ease severe agitation, aggressive outbursts, and hallucinations. But they come with risks, including a potential for weight gain and diabetes. Also, antipsychotics now carry a warning that they are associated with an increased risk of death in seniors with dementia.
Four of the five Alzheimer's drugs — donepezil (Aricept), galantamine (Razadyne), rivastigmine (Exelon), and tacrine (Cognex) — belong to the same class and essentially work the same way. They reduce the breakdown in the brain of a chemical called acetylcholine, which is a chemical messenger that transmits information from nerve cell to nerve cell. This effectively increases levels of acetylcholine in the brain, and may preserve brain function. The problem is that the brain cells that make and release acetylcholine are slowly being destroyed by the disease and so eventually these types of drugs can no longer be effective.
The fifth and most recently approved drug, memantine (Namenda), works differently. It blocks the actions of the neurotransmitter, glutamate. Glutamate is needed for memory, but too much of it is toxic to nerve cells, and it appears that in people with Alzheimer's, there is too much of it (for unknown reasons).
None of these five drugs "cure" Alzheimer's disease. Instead, they are intended to slow a person's mental decline and ease symptoms. As Table 2 shows, on average, the medications slightly slowed cognitive decline compared to placebo and slightly improved people's ability to perform daily activities, but these effects were very small and are not considered to be meaningful improvements. In addition, most people experienced side effects, and many stopped taking the medication because they could not tolerate the adverse effects (especially forgetfulness and confusion).
However, studies indicate that when people taking any of the Alzheimer's medicines are compared to those taking a placebo, most do not get a meaningful benefit with marked improvement, or a significant delay in the worsening of symptoms.
By another measure, one team of researchers calculated that for every three to seven people taking an Alzheimer's drug, only one benefits at all. Unfortunately, there is no way, as of yet, to predict who will respond, and who will have little or no benefit.

Notably, some studies indicate that the general health of elderly people who take these medicines does not decline as rapidly, an indication they may have benefits other than those assessed by tests of mental function and memory.
Thus, the decision by a doctor, the Alzheimer's patient, and his or her loved ones, is whether the medication is worth trying.
Given the poor to modest benefits of these drugs, you might want to consider four factors to help you assess whether treatment is worth it:
Taking these factors one by one:
Symptoms and maintaining brain function: Strong motivation to maintain independence and brain function is likely to compel many newly diagnosed Alzheimer's patients to try one of the medications, at least for a while. All of the drugs, except memantine (Namenda), are indicated for early-stage patients, and they are considered to be most effective when given in the early stages. But if symptoms are mild or moderate, a response in the early stages may be hard to notice. Therefore, the gamble is that the drug will work to delay decline and worsening of symptoms, and that a strong response will occur. It may or may not; every person is different.
Also, as the disease progresses, people often seem to get less benefit from any of the Alzheimer's drugs. The benefit they may have experienced in the early stages appears to wane or disappear.
Side effects: One Alzheimer's medication, tacrine (Cognex), should be avoided, because it can cause liver damage, so its risks outweigh any potential benefits. In studies, as many as half of people who took Cognex had abnormal liver functions. Because of this, Cognex is now prescribed only rarely.
The four remaining drugs can cause minor side effects, and, for some people, these outweigh the sometimes limited benefits. (Table 3 lists the primary possible side effects.) In the next section we discuss how the drugs vary with respect to the side effects they cause. The relevant point here is that 20 to 25 percent of people stop taking an Alzheimer's drug because of side effects. The effects generally go away when the drug is stopped.
Table 2: Possible Side Effects
Relatively Minor: Usually go away in time or are short-lived |
|
Potentially Severe: Can be annoying or dangerous and should be reported to a doctor |
|
Taking the drugs long-term: Any potential benefits of the Alzheimer's drugs only occur while they are being taken. When the drugs are stopped, mental function might decline. Very few studies have examined the long-term effectiveness and safety of these medicines. Most of the studies assessed people's response for just six months, though a few have suggested the benefits might last one to three years.
While there is no proof of long-term problems, there is concern that some might occur. For example, some researchers believe the Alzheimer's drugs might cause a slowed heart rate over time. This can cause dizziness and lead to falls—a potentially dangerous situation in someone who might already be unsteady on their feet. While the evidence so far indicates the heart rate reduction is minimal, more research is needed. There is also the complication that tens of thousands of older people have undiagnosed heart failure (also called congestive heart failure), and a slowed heart rate could be dangerous for them. Doctors usually—but may not always—assess this before prescribing an Alzheimer's drug.
In addition, research indicates that, with the exception of Namenda, all the Alzheimer's drugs can increase the risk of stomach bleeding and ulcers when taken for a long time.
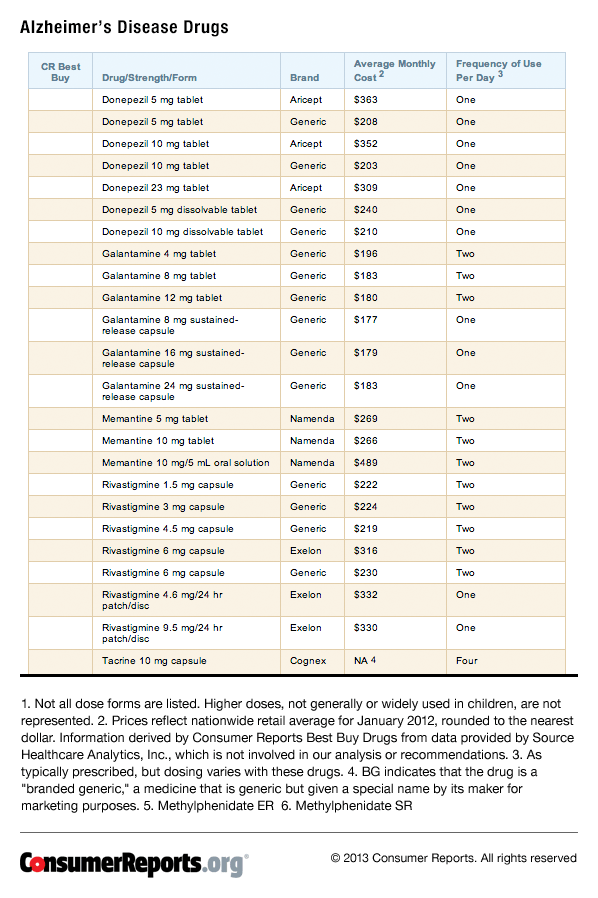
Cost and coverage: The Alzheimer's drugs are relatively expensive, ranging in cost from $177 to more than $400 per month. What you'll pay will depend, of course, on your drug insurance coverage. The drugs are now covered under the Medicare Part D drug benefit program. But you could still end up paying quite a lot out-of-pocket for these medicines, due to co-payments and the structure of the Medicare drug benefit. For example, if the total amount of your drug costs during one year exceeds $2,950 you'll hit what's dubbed the "donut hole." As of press time, once you hit the donut hole you'll then pay for half the cost of branded drugs.
Indeed, for many Alzheimer's patients and their families, the decision of whether to take an Alzheimer's drug may be based, in part, on the expense of the other medications they might take for other conditions. In addition, many Alzheimer's patients take an antidepressant or other drug to help ease their Alzheimer's symptoms. For low-income and middle-income Alzheimer's patients, the burden of the cost for other medicines can simply be too great to justify another drug with a high co-payment if there is only a marginal chance the drug will bring them any benefit.
The five Alzheimer's drugs are roughly equivalent in their effectiveness, with no clear evidence that any one is more effective than the others. They do differ in the side effects they cause. Cost is also an important factor for many people, and some Alzheimer's medications are available as generics, which generally have a substantially lower cost than brand name counterparts (See Table 4 below).
Of the five available Alzheimer's medications, tacrine (Cognex) is ruled out due to the risk it poses to the liver. Your doctor is unlikely to prescribe this drug be cause it has been linked to liver damage. Should it be recommended, we advise a thorough conversation with your doctor about the choice, and a second opinion.
As mentioned earlier, all of the Alzheimer's drugs, except memantine (Namenda), are indicated for treatment early in the course of the disease. Donepezil is the only generic drug specifically approved by the FDA for treatment of middle-stage and late-stage
Alzheimer's (Namenda is also approved for middle-stage and late-stage but it is not available as a generic), although the other medicines are also commonly prescribed for people with later-stage disease.
Namenda is not approved to treat early-stage Alzheimer's. Indeed, studies have not shown it to be effective for such patients.
That means you and your doctor have a choice of three drugs intended for early use: donepezil (Aricept), galantamine (Razadyne), and rivastigmine (Exelon). Of these three, rivastigmine had the highest incidence of several side effects when compared to both placebo and in studies that compared it to the other two. For example, in one analysis, 20 percent of people taking rivastigmine had nausea and vomiting, compared to 13 percent of those taking galantamine and 7 percent taking donepezil.
Likewise, people taking rivastigmine also had higher rates of weight loss and dizziness compared to those taking donepezil or galantamine. Donepezil had the lowest rate of both these side effects in several studies. It should be noted, however, that other analyses failed to find statistically significant side effect differences among these three drugs.
An important measure of any drug is how often patients stop taking it because of side effects. Rivastigmine rates lower on this factor, too. In one study, 22 percent of patients stopped taking it compared to 11 percent of those taking donepezil. Donepezil might offer an advantage over galantamine in the short-term. In one 12-week study comparing the medications, more patients stopped taking galantamine. But in a year-long study, an equal number (13 percent) stopped taking each drug.
However, we realize that many people will want to try one of these medications if somebody they care for is suffering from Alzheimer's disease. In that case, it makes sense to try one that has the lowest rate of side effects and is the least expensive since none of the medications has been shown to offer superior effectiveness. Generic donepezil or generic galantamine meet both criteria. Both have a lower risk of adverse effects and higher tolerability than the other medications, and since they are generic, their price is significantly less than brand-name options.
But, again, we emphasize the effectiveness of those two drugs (and indeed any Alzheimer's medication) may be marginal at best in most people who try them. If there are no indications of improvement after three months, it is unlikely that there ever will be, so the drug should then be stopped.
Namenda is sometimes prescribed in combination with one of the other Alzheimer's drugs in people with middle- to late-stage disease. However, it's unclear if there's an increased benefit from taking two medicines. In fact, a recent study published in the March 8, 2012 issue of the New England Journal of Medicine found the combination of Namenda and donepezil was no better than donepezil alone in people with moderate-to-severe Alzheimer's disease. Also, the combined treatment doubles the cost and could increase your risk of side effects.
Donepezil and galantamine—as with the other Alzheimer's medicines—are usually prescribed at the lowest dose available when you first begin taking them. That dose is then doubled after a few weeks or months, depending on how you are tolerating the drug. And if that additional dose is also well-tolerated, the dose may be increased again.
Importantly, because all the Alzheimer's drugs, except Namenda, work in the same way, switching from one to another usually does not produce significantly different results. However, that said, some patients do respond better to one drug than another, or may tolerate one better than another. e drug will bring them any benefit.

The benefits of these drugs are usually assessed based on the following criteria:
All five drugs have been shown to slow cognitive decline, but usually only slightly. All have also been found to generally improve a patient's overall response to treatment — both nondrug and using a variety of medicines to control or ease symptoms (such as antidepressants or anti-anxiety drugs).
Our analysis found inconsistent evidence on how these drugs compare in their ability to improve patients' daily functioning, behavior, or quality of life. A few of the drugs have been studied more extensively than others in these areas. Unfortunately, few studies directly, and comprehensively, compared one drug to another, which leaves open the question of whether any one is superior in effectiveness to the others.
Cognitive Ability
Most of the evidence, however, suggests that there's no substantial difference among the five drugs in their ability to produce improvements in memory and other cognitive functions. Compared to placebo, all five are capable of yielding noticeable changes in cognitive ability between two to six weeks after treatment starts.
But, as stated earlier, most people who take the medications, versus those who take a placebo, do not show a meaningful improvement in cognitive ability.
Overall Improvement
To assess whether patients are improving overall—known as global response to treatment—studies rely on the opinions of the treating physician and the caregiver, often a family member.
In general, all of the Alzheimer's medicines have been shown to improve a patient's well-being. However, most patients will not have this improvement. Studies have shown that only about 10 percent of patients are considered to be "better" when assessed by their doctor or caregiver. Although others may not decline as much as they would have without drug treatment, overall improvement likely will not be noted for most patients.
There were no high quality studies comparing the different treatments head-to-head in their ability to improve overall well-being. Several studies evaluated physician and caregiver satisfaction with a drug when compared with another. Two compared donepezil (Aricept) to galantamine (Razadyne), and one compared donepezil to rivastigmine (Exelon). In two of these studies, donepezil performed somewhat better.
Daily Functioning
Daily functioning refers to the ability to perform normal activities of daily living, such as getting dressed, preparing food, shopping, housekeeping, or handling money. In the early stages of the disease, it is more difficult to determine whether a treatment can significantly affect these abilities. But as the disease progresses, this measure becomes more useful in assessing a drug's impact.
Daily functioning has not been measured in every study. And studies where it has been used have produced inconsistent results, partly because improvement can vary so much among people.
For example, donepezil (Aricept) was found to improve daily functioning in two studies, but not in three others; three galantamine (Razadyne) studies found improvement in daily functioning while two others did not. And in one year-long study comparing these two drugs, there was no difference between them.
Behavior
Evaluation of behavior may be more relevant in people with severe Alzheimer's disease, and since most of the drugs have been studied in earlier stages of the illness, behavior was not always measured.
The drugs' impact on behavior was assessed in seven studies: four with donepezil (Aricept), two with galantamine (Razadyne), and one with memantine (Namenda). Compared to no treatment, these drugs produced only minimal improvements. There was only one study that compared two drugs' effects on patients' behavior. In that year-long trial, there was no significant difference between donepezil and galantamine.
Table 3: Effectiveness of Alzheimer's Medications
Generic name |
Brand name |
Slowing of cognitive decline |
Activities of daily living |
Overall risk of adverse events |
Stopped taking medication because of adverse events |
Comments/ special notes |
| Donepezil | Aricept | Very small effect* | Very small effect + | Up to 88% | On average 19% | Risk of muscle cramps and insomnia |
| Galantamine | Razadyne | Very small effect* | Very small effect + | Up to 90% | On average 17% | |
| Memantine | Namenda | Very small effect* | Very small effect + | Up to 84% | Up to 10% | |
| Rivastigmine | Exelon | Very small effect* | Very small effect + | Up to 96% | On average 24% | High risk of nausea, vomiting, gastro-intestinal related events |
| Tacrine | Cognex | Data not reported | Data not reported | Up to 51% | On average 43% | Risk of liver toxicity |
* Compared with placebo differences were smaller than 4 points on the Alzheimer's Disease Assessment Scale-cognitive subscale (ADAScog), which is so small, it is not considered clinically meaningful.
+ Measured by caregivers on the Progressive Deterioration Scale (PDS), compared with placebo. On average, the small improvements seen on this scale in people taking an Alzheimer's medication would not be considered clinically meaningful by most doctors.
Quality of life
There is scant evidence that the Alzheimer's drugs improve patients' quality of life. Seven studies — six of donepezil (Aricept) and one of tacrine (Cognex) — compared those drugs to placebo. Aricept appeared to significantly improve patients' quality of life in two of the studies, but they only lasted 12 to 15 weeks. There are no studies that compare one drug to another in the area of quality of life.
Age, Race, and Gender Differences
Most Alzheimer's drug studies have been conducted in people who are 70 to 75 years old. Although some studies have tested Alzheimer's drugs in older people or more critically ill people who live in nursing homes, none has been able to determine if age makes a difference in how well the drugs work, or if they are more or less safe.
No direct evidence suggests that any Alzheimer's drug has better or worse efficacy for any gender or racial group.
Safety
The Alzheimer's medications can cause several side effects. While the most common are minor and might be short-lived, for some people, they might persist or be intolerable. These medications can also pose more serious side effects in rare cases.
Commonly reported adverse events in clinical studies include gastrointestinal side effects (nausea, vomiting, diarrhea), dizziness, fatigue, insomnia, loss of appetite, muscle cramps, tremor, vertigo, and weight loss. Nausea and vomiting occurred frequently in trials of donepezil, galantamine, and rivastigmine. Between 10 to 50 percent of people who took one of those medications experienced nausea or vomiting. Studies also suggest that side effects are more likely to occur when a person is taking a high dose of these medications.
The available evidence suggests that there might be some differences in side effect profiles of the Alzheimer's medications. Studies indicate that galantamine and rivastigmine pose a higher risk of gastrointestinal side effects than donepezil. Memantine might carry a lower risk of side effects than the other medications. In one study, the only adverse events reported by more than 10 percent of people who took memantine included agitation, diarrhea, drowsiness, and urinary incontinence. Overall, no side effect was reported more in memantine-treated people than in those who took a placebo pill.
The most serious safety concern in this drug class is the liver toxicity associated with tacrine (Cognex), which, as we previously noted, is now rarely prescribed because of this risk. Liver toxicity has not been reported for donepezil, galantamine, rivastigmine, or memantine.
Other rare but potentially serious adverse events associated with Alzheimer's medications include cardiovascular problems, such as a slower than normal heartbeat, and a condition called heart block. These medications can also cause gastrointestinal bleeding and ulcers. The labeling of these drugs cautions that they might have the potential to cause convulsions or seizures.
Drug Interactions
Although Alzheimer's drugs are generally safe, they may interact with other medicines or dietary supplements in ways that can be dangerous. Be sure to tell your doctor about all the other medications you are taking, including all vitamins and herbal therapies.
The main drugs to be concerned about are:
It may occasionally be acceptable to take some of the medications together. Your doctor or pharmacist can tell you when combining the medications is appropriate or safe.
Certain other medications may interact with Alzheimer's drugs. Make sure to ask your doctor or pharmacist before taking any new prescription or over-the-counter medications.
Based on the evidence of their effectiveness, side effects, tolerability, flexibility of use, and cost, we are unable to choose any of the Alzheimer's medications as Consumer Reports Best Buy Drugs selections. The available studies indicate that most people will receive no benefit at all from taking a medication. Balanced against their relatively high price tag and the risk of side effects, including rare but serious safety concerns, we cannot endorse any of the medications.
This is a change from our previous version of this report published in 2006, which selected Best Buys. However, since then, several systematic reviews of multiple studies have been published that have found little, if any, benefit from Alzheimer's medications.
These include a 2007 study published in Public Library of Science Medicine, which concluded that the medications were "not associated with any delay in the onset of Alzheimer's disease" and also expressed concern about the side effects; treatment guidelines from the American College of Physicians, first published in 2008, which state,
"All of the drugs have known adverse events, and the decision to manage patients with dementia should balance harms against modest or even no benefit"; and a 2010 review by the Agency for Healthcare Research and Quality, which concluded these medications have no effect on delaying the onset of Alzheimer's or improving or maintaining cognitive function.

Our evaluation is primarily based on an independent scientific review of the evidence on the effectiveness, safety and adverse effects of the Alzheimer's drugs. A team of physicians and researchers at Oregon Health & Science University Evidence-based Practice Center con ducted the analysis as part of the Drug Effectiveness Review Project, or DERP. DERP is a first-of-its-kind multi-state initiative to evaluate the comparative effectiveness and safety of hundreds of prescription drugs.
A synopsis of DERP's analysis of the Alzheimer's drugs forms the basis for this report. A consultant to Consumer Reports Best Buy Drugs is also a member of the Oregon-based research team, which has no financial interest in any pharmaceutical company or product.
The full DERP review of the Alzheimer's drugs is available at https://derp.ohsu.edu/about/final-document-display.cfm. (This is a long and technical document written for physicians.)
Our analysis also took into account a 2010 evidence report conducted by the Agency for Healthcare Research and Quality (AHRQ), and an evaluation conducted by the Department of Veteran's Affairs Pharmacy Benefits Management Strategic Health Group. This can be obtained at www.vapbm.org.
The drug costs we cite were obtained from a healthcare information company which tracks the sales of prescription drugs in the U.S. Prices for a drug can vary quite widely, even within a single city or town. All the prices in this report are national averages based on sales of prescription drugs in retail outlets. They reflect the cash price paid for a month's supply of each drug in January 2012.
Consumer Reports selected the Best Buy Drugs using the following criteria. The drug had to:
-Be approved by the FDA for treating Alzheimer's disease.
-Have a safety record equal to or better than other Alzheimer's drugs.
-Have an average price for a 30-day supply that was not higher than the other Alzheimer's drugs.
The Consumers Reports Best Buy Drugs methodology is described in more detail in the Methods section at www.CRBestBuyDrugs.org.
Agid Y, Dubois B, Anand R, Gharabawi G. Efficacy and tolerability of rivastigmine in patients with dementia of the Alzheimer type. Current Therapeutic Research, Clinical & Experimental 1998; 59(12):837-45.
Areosa S, Sherriff F. Memantine for dementia. Cochrane Database Syst Rev 2004; (1):CD003154.
Birks J, Grimley Evans J, Iakovidou V, Tsolaki M. Rivastigmine for Alzheimer's disease. Cochrane Database Syst Rev 2004; (4):CD001191.
Birks J, Harvey R. Donepezil for dementia due to Alzheimer's disease. Cochrane Database Syst Rev 2004; (3):CD001190.
Burns A, Rossor M, Hecker J et al. The effects of donepezil in Alzheimer's disease - results from a multinational trial. Dement Geriatr Cogn Disord 1999; 10(3):237- 44.
Burns A, Spiegel R, Quarg P. Efficacy of rivastigmine in subjects with moderately severe Alzheimer's disease. Int J Geriatr Psych. 2004; 19(3):243-9.
Corey-Bloom J, Anand J, Veach J, and E7BSG. A randomized trial evaluating the efficacy and safety of ENA 713 (rivastigmine tartrate), a new acetylcholinesterase inhibitor, in patients with mild to moderately severe Alzheimer's disease. International Journal of Geriatric Psychopharmacology 1998; 1:55-65.
Doody RS, Ferris SH, Salloway S, et al. Donepezil treatment of patients with MCI: a 48-week randomized, placebo-controlled trial. Neurology 2009;72(18):1555-61.
Dunn N, Pearce G, Shakir S. Adverse effects associated with the use of donepezil in general practice in England. J Psychopharmacol 2000; 14(4):406-8.
Farlow M, Brashear A, Hui S, Schneider L, Unverzagt F, and TSG. The effects of tacrine in patients with mild versus moderate stage Alzheimer's disease. Research Advances in Alzheimer's Disease and Related Disorders 1995; 283-92.
Farlow M, Gracon S, Hershey L, Lewis K, Sadowsky C, Dolan-Ureno J. A controlled trial of tacrine in Alzheimer's disease. The Tacrine Study Group. JAMA 1992; 268(18):2523-9.
Feldman H, Gauthier S, Hecker J, Vellas B, Subbiah P, Whalen E . A 24-week, randomized, doubleblind study of donepezil in moderate to severe Alzheimer's disease. Neurology 2001; 57(4):613-20.
Homma A, Takeda M, Imai Y et al. Clinical efficacy and safety of donepezil on cognitive and global function in patients with Alzheimer's disease. A 24-week, multicenter, double-blind, placebocontrolled study in Japan. E2020 Study Group. Dement Geriatr Cogn Disord 2000; 11(6):299-313.
Jones R, Soininen H, Hager K et al. A multinational, randomised, 12-week study comparing the effects of donepezil and galantamine in patients with mild to moderate Alzheimer's disease. Int J Geriatr Psychiatry 2004; 19(1):58-67.
Knapp M, Knopman D, Solomon P, Pendlebury W, Davis C, Gracon S. A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer's disease. The Tacrine Study Group. JAMA 1994; 271(13):985-91.
Knopman D, Schneider L, Davis K et al. Long-term tacrine (Cognex) treatment: effects on nursing home placement and mortality, Tacrine Study Group. Neurology 1996; 47(1):166-77.
Koontz J, Baskys A. Effects of galantamine on working memory and global functioning in patients with mild cognitive impairment: a double-blind placebo-controlled study. Am J Alzheimers Dis Other Demen 2005;20(5):295-302.
Lanctot K, Herrmann N, Yau K et al. Efficacy and safety of cholinesterase inhibitors in Alzheimer's disease: a meta-analysis. CMAJ 2003; 169(6):557-64.
Mohs R, Doody R, Morris J et al. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology 2001; 57(3):481-8.
Molloy, W. & Caldwell, P., Alzheimer's Disease: Everything You Need to Know — Revised Edition (2003); Firefly Books, Buffalo, NY.
Olin J, Schneider L. Galantamine for Alzheimer's disease. Cochrane Database Syst Rev 2004; (3):CD001747.
Peterson, Ronald (editor), Mayo Clinic on Alzheimers Disease (2002); Mayo Clinic Health Information, Rochester, MN/Kensington Publishing Corp, NY.
Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med 2005;352(23):2379-88.
Potkin S, Anand R, Hartman R, Veach J, Grossberg G. Impact of Alzheimer's disease and rivastigmine treatment on activities of daily living over the course of mild to moderately severe disease. Prog Neuropsychopharmacol Biol Psychiatry 2002; 26(4):713-20.
Qizilbash N, Birks J, Lopez Arrieta J, Lewington S, Szeto S. Tacrine for Alzheimer's disease. Cochrane Database Syst Rev 2000; (3):CD000202.
Raschetti R, Albanese E, Vanacore N, et al. Cholinesterase inhibitors in mild cognitive impairment: a systematic review of randomised trials. PLoS Medicine / Public Library of Science 2007;4(11):e338.
Raskind M, Peskind E, Wessel T, Yuan W. Galantamine in AD: A 6-month randomized, placebocontrolled trial with a 6-month extension. The Galantamine USA- 1 Study Group. Neurology 2000; 54(12):2261-8.
Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius H. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med 2003; 348(14):1333-41.
Rogers S, Doody R, Mohs R, Friedhoff L. Donepezil improves cognition and global function in Alzheimer disease: a 15-week, double-blind, placebo-controlled study. Donepezil Study Group. Arch Intern Med 1998; 158(9):1021-31.
Rogers S, Farlow M, Doody R, Mohs R, Friedhoff L. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer's disease. Donepezil Study Group.[see comment]. Neurology 1998; 50(1):136-45.
Rogers S, Friedhoff L. The efficacy and safety of donepezil in patients with Alzheimer's disease: results of a US Multicentre, Randomized, Double-Blind, Placebo-Controlled Trial. The Donepezil Study Group. Dementia 1996; 7(6):293-303.
Rosler M, Anand R, Cicin-Sain A et al. Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomised controlled trial. BMJ 1999; 318(7184):633-8.
Salloway S, Ferris S, Kluger A, et al. Efficacy of donepezil in mild cognitive impairment: a randomized placebo-controlled trial. Neurology 2004;63(4):651-7.
Schneider L. AD2000: donepezil in Alzheimer's disease. Lancet 2004; 363(9427):2100-1.
Tariot P, Cummings J, Katz I et al. A randomized, double-blind, placebo-controlled study of the efficacy and safety of donepezil in patients with Alzheimer's disease in the nursing home setting. J Am Geriatr Soc 2001; 49(12):1590-9.
Tariot P, Farlow M, Grossberg G, Graham S, McDonald S, Gergel I. Memantinetreatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA 2004; 291(3):317-24.
Tariot P, Solomon P, Morris J, Kershaw P, Lilienfeld S, Ding C. A 5-month, randomized, placebocontrolled trial of galantamine in AD. The Galantamine USA- 10 Study Group. Neurology 2000; 54(12):2269-76.
Watkins P, Zimmerman H, Knapp M, Gracon S, Lewis K. Hepatotoxic effects oftacrine administration in patients with Alzheimer's disease. JAMA 1994; 271(13):992-8.
Whitehead A, Perdomo C, Pratt RD, Birks J, Wilcock GK, Evans JG. Donepezil for the symptomatic treatment of patients with mild to moderate Alzheimer's disease: a meta-analysis of individual patient data from randomised controlled trials. Int J Geriatr Psychiatry 2004; 19(7):624-33.
Wilcock G, Howe I, Coles H et al. A long-term comparison of galantamine and donepezil in the treatment of Alzheimer's disease. Drugs Aging 2003; 20(10):777-89.
Wilcock G, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer's disease: multicentre randomised controlled trial. Galantamine International-1 Study Group. BMJ 2000; 321(7274):1445-9.
Wilkinson D, Passmore A, Bullock R et al. A multinational, randomised, 12-week, comparative study of donepezil and rivastigmine in patients with mild to moderate Alzheimer's disease. Int J Clin Pract 2002; 56(6):441-6.
Williams JW, Plassman BL, Burke J, Holsinger T, Benjamin S. Preventing Alzheimer's Disease and Cognitive Decline. Evidence Report/Technology Assessment No. 193. (Prepared by the Duke Evidence-based Practice Center under Contract No. HHSA 290-2007-10066-I.) AHRQ Publication No. 10-E005. Rockville, MD: Agency for Healthcare Research and Quality. April 2010.
Winblad B, Engedal K, Soininen H et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology 2001; 57(3):489-95.
Winblad B, Gauthier S, Scinto L, et al. Safety and efficacy of galantamine in subjects with mild cognitive impairment. Neurology 2008;70(22):2024-35.
Wood P, Castleden C. A double-blind, placebo controlled, multicentre study of tacrine for Alzheimer's disease. Int. J Geriatr. Psych 1994; 9(8):649-54.
Yesavage JA, Friedman L, Ashford JW, et al. Acetylcholinesterase inhibitor in combination with cognitive training in older adults. J Gerontol B Psychol Sci Soc Sci 2008;63(5):P288-94.
These materials are made possible by a grant from the state Attorney General Consumer and Prescriber Education Grant Program, which is funded by the multi-state settlement of consumer-fraud claims regarding the marketing of the prescription drug Neurontin (gabapentin).
 WASHING MACHINE REVIEWS
WASHING MACHINE REVIEWS GENERATOR REVIEWS
GENERATOR REVIEWS
 Build & Buy Car Buying Service
Build & Buy Car Buying Service
Save thousands off MSRP with upfront dealer pricing information and a transparent car buying experience.
 Get Ratings on the go and compare
Get Ratings on the go and compare
while you shop